
What does he have to offer a well-heeled multi-national company looking to capitalize in the US?
Is it the beauty device that smooths out cellulite?
The shockwave medical device that heals wounds and injuries?
The low-cost medical device that can handle any mutant strain of malaria?
Mr. Hague tells me his Cellsonic machines do it all – but none of these indications are FDA cleared – and, without a predicate device on the market, trials will be very, very expensive.
Andrew explained the opportunity and outlined what he needs to do next.
Joe Hage: Welcome to #MedDevice. We’ve moved up the time to accommodate our UK-based guest, Andrew Hague. Welcome Andrew! Tell us about Cellsonic.
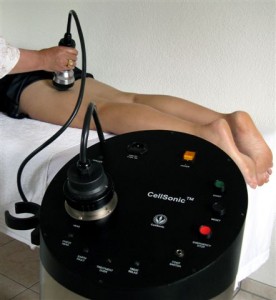
Andrew Hague: CellSonic makes many machines. The star product is a hand-held, shockwave machine that heals wounds, injuries and smooths out cellulite. It also removes fat. So we are in the medical business and the beauty business.
Joe Hage: One of the things I most enjoy about running the Medical Devices Group on LinkedIn is I get to meet interesting folks like you. You were telling me your products are CE marked but have not been cleared for use in the US.
Joe Hage: You could put each of these indications into one 510(k) application, though, yes?
Andrew Hague: We are not sure because the CellSonic is a Class II machine and we do not know if it possible for this class.
Joe Hage: I’m not a regulatory expert but I’m confident some of my readers are. (If you are an FDA expert, please confirm: The 510(k) process works for Class II devices, yes?)
In the meanwhile, let’s assume an FDA expert responds to this conversation and says, yes, they can help you file your Class II.
Andrew Hague: However, we do not know of another shockwave machine in whose footsteps we can follow. I believe the 510(k) requires a predicate device.
Joe Hage: I believe the sticking point is having trials if you don’t have a predicate device. That’s what would hold up your 510(k).
This tension explains some of the conversation over on the LinkedIn thread where we met, “Is a CE mark good enough?”
Andrew Hague: Yes, without competitors, we have no predicate device so we have to be pioneers. We are looking for a big company which can pay for a lot of FDA trials.
They must also have a big sales force for when permission is recorded there will be a big demand.

The CellSonic Medical machine is a lithotripter with variable power for use in hospitals and clinics to treat bones, wounds, and sports injuries. (Click for more.)
Andrew Hague:A lithotripter breaks a kidney stone noninvasively with sudden acoustic bangs into grains of sand to pee out. On the question of a business partner, we may need two: One for medical, one for beauty.
The market for diabetic foot ulcers is one person in 1000. For cellulite, it is virtually any woman over the age of 30.
Joe Hage: How long has Cellsonic been in business? How did you develop this device?
Andrew Hague: I began with the first lithotripter in England in 1987. Later I was the distributor for a Swiss machine. About ten years ago came the opportunity to make our own machines and only now do we have the CellSonic ready to sell. These are complicated machines to make and easy to use. Ours is half the price of anything similar from China.
Joe Hage: So you do have competitors?
Andrew Hague: We have potential competitors from China. What I hear about their products is, they don’t work. But I am sure, before long, they will work.
Joe Hage: Is this on the beauty side, the medical side, or both?
Andrew Hague: Both. Our clever design will enable us to always be the least expensive.
Joe Hage: OK, I’ll bite. How can you be confident you will always be the least expensive?
Andrew Hague: The design helps keep the cost down and you can’t get cheaper than where we make it, in India.
Now, cosmetic treatments for cellulite may not be terribly price sensitive in America. But if you consider the medical side, this device could potentially help millions with buruli ulcer in Africa and chagas in Latin America, for example.
Malaria kills millions and our device can handle any mutant strain because, instead of treating it pharmacologically, we are mechanically hitting the germ.
When the shockwave travels through the cell, it stretches it four times per second and it comes back under its own elasticity until it eventually ruptures. The small things like viruses, bacteria, and parasites are exploded.
So you can see, being low cost is very important when you consider the third-world markets we can affect.
Joe Hage: Someone will ask, “The poor people have no money. How will you make a profit at that?” And you will say …

Will the UN help?
Andrew Hague:The money is in Geneva. The United Nations, WHO, Unicef, and Medecins sans Frontiere are already spending more than we need to do our job.
Geneva will only listen to its operators in the field. So we need to convince the NGOs that this new technology is better than what is not working properly at present.
Joe Hage: Ok. I follow you. If Geneva and the third world are the primary markets for the medical side, why do you need to partner with a big-heeled multi-national? Why aren’t you approaching Geneva yourself?
Andrew Hague: Outside the US, we don’t need a big partner.
Joe Hage: Then have you already approached Geneva? What was their response?
Andrew Hague: Geneva only listens to workers in the field. So we are now getting the message to West Africa, Ecuador, Peru, and the message will feed back to Geneva.
Joe Hage: Got it. You know, Andrew, this is fascinating to me. I fear this interview is getting long but I have to ask, on a very separate note, you were telling me some intriguing facts about cellulite I had never heard before.
Cellulite is a junk yard for body waste and can only be removed by loosening it and letting the waste escape by encouraging the growth of capillary veins. (More)

Andrew Hague:Cellulite is fat without blood so it cannot be accessed by the digestion system as a reserve fuel. Women get cellulite at the back of the legs. In men it is at the side of the waist, love handles. If you starve, you will still have cellulite. Athletes will get cellulite unless they are very careful.
Joe Hage: How do “men of a certain age” know if they have fat or cellulite around their waists? And what does “very careful” mean in this context?
Andrew Hague: If you don’t know you obviously have both so starve (diet) and see what’s left, that’s the cellulite. It means not being in civilised society. The answer for health is not medicine but transport.
Andrew Hague: Remember we are all cave men. Mechanised transport is a major problem. Walk, run, or cycle and you have all the exercise you need. Ban cars.
Joe Hage: Talk us through your next steps. How, specifically, will you approach beauty and/or medical multi-nationals for FDA clearance?
Andrew Hague: Simultaneously. They are aiming at 10% return on capital and both markets are big enough.
Joe Hage: Let’s use this interview as an opportunity to get your message out there. What should an interested party do? You have the floor, Sir.
Andrew Hague: The US market is unique so it is best run by Americans. The owners could be non-US but surely this is for America. We would create an American company and it would serve American people making profits in America.
Selling to doctors is, of course, different than selling to beauty salons so that’s why separate companies may be best. The next step for CellSonic is treating blood for transfusions. All germs will be killed and the blood safe.
Being able to clean blood leads to cleaning blood in the patient which could cure malaria, hepatitis, and maybe HIV – without drugs.
This means we are competitors to Big Pharma because we solve their problems and do not use drugs. There will always be a need for drugs but now there is a cheaper alternative without side effects.
Joe Hage: Andrew, this has absolutely been one of the most enlightening #MedDevice chats I’ve ever had. Thank you so much.
Andrew Hague: My task is to find the right partners for many years to come. CellSonic will outlive me.
Joe Hage: Yes, unless you live for generations and generations! I wish you the very best, Andrew. Thanks again for talking with us today.
…
>>> Click to review the archive of #MedDevice chats you missed! <<<
Join the Medical Devices Group on LinkedIn to network and grow your business. #MedDevice is held most Wednesdays, 4 p.m. EST. We interview the medical device industry’s best minds and cover issues including sales, reimbursement, distribution, EMRs/HIT, regulations, and Marketing Medical Devices.











